Organic Chemistry Class 11 MCQ is one of the best strategies to prepare for the CBSE Class 11 Board exam. If you want to complete a grasp concept or work on one’s score, there is no method except constant practice. Students can improve their speed and accuracy by doing more MCQs on Organic Chemistry Class 11, which will help them all through their board tests.
Organic Chemistry Class 11 MCQ Question with Answer
Class 11 Chemistry MCQ with answers are given here to Chapter 12 Organic Chemistry. These MCQs are based on the latest CBSE board syllabus and relate to the latest Class 11 Chemistry syllabus. By Solving these Class 11 MCQs, you will be able to analyze all of the concepts quickly in the chapter and get ready for the Class 11 Annual exam.
Learn Class 11 Organic ChemistryMCQs with answers pdf free download according to the latest CBSE and NCERT syllabus. Students should prepare for the examination by solving CBSE Class 11 Chemistry Organic Chemistry MCQ with answers given below
Question 1. The absolute configuration of the following
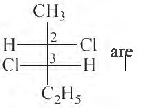
(a) 2S, 3R
(b) 2S, 3S
(c) 2R, 3S
(d) 2R, 3R
Answer
B
Question 2. In laboratory, first organic compound was synthesised by
(a) Kekule
(b) Hennel
(c) Wohler
(d) Liebig
Answer
C
Question 3. Which of the following organic compound was synthesised by F. Wohler from an inorganic compound?
(a) Methane
(b) Urea
(c) Acetic acid
(d) Chloroform
Answer
B
Question 4. The discovery that shook the belief in the vital force theory was
(a) Stereoisomerism
(b) Synthesis of indigo
(c) Wholer’s synthesis of urea from ammonium cyanate
(d) Fermentation of sugars
Answer
C
Question 5. Who is known as the “Father of Chemistry”?
(a) Faraday
(b) Priestley
(c) Rutherford
(d) Lavoisier
Answer
D
Question 6. The percentage of s- character of the hybrid orbitals in ethane, ethene and ethyne are respectively.
(a) 50, 75, 100
(b) 10, 20, 40
(c) 25, 33, 50
(d) 25, 50, 75
Answer
C
Question 7. Which of the following scientist proposed that a ‘vital force’ was responsible for the formation of organic compounds ?
(a) Berzilius
(b) Wohler
(c) Berthelot
(d) Kolbe
Answer
A
Question 8. Which of the following molecules is expected to rotate the plane of plane polarised light?
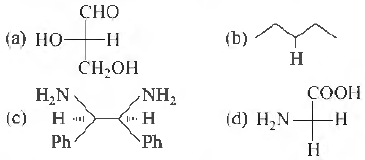
Question 9. First organic compound to be synthesised was
(a) methane
(b) cane sugar
(c) acetic acid
(d) urea
Answer
A
Question 10. 2- Pentene contains
(a) 15 σ- and one π- bond
(b) 14 σ-and one π- bond
(c) 15 σ- and two π- bonds
(d) 14 σ- and two π- bonds
Answer
B
Question 11. The successive members in a homologues series differ from each other by ________
(a) – CH2CH2 – unit
(b) – CH2 unit
(c) – OCH3 unit
(d) – CH3 unit
Answer
B
Question 12. Tautomerism is exhibited by
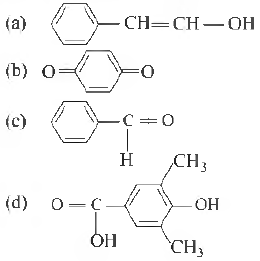
Answer
A
Question 13. Select the molecule which has only one π-bond
(a) CH ≡ CH
(b) CH2 = CHCHO
(c) CH3CH = CH2
(d) CH3CH=CHCOOH
Answer
C
Question 14. In the hydrocarbon CH3 – CH = CH – CH2 – C ≡ CH
6 5 4 3 2 1
The state of hybrization of carbons 1, 3 and 5 are in the following sequence
(a) sp2, sp, sp3
(b) sp, sp3, sp2
(c) sp, sp2, sp3
(d) sp3, sp2, sp
Answer
B
Question 15. Among the following four structures I to IV
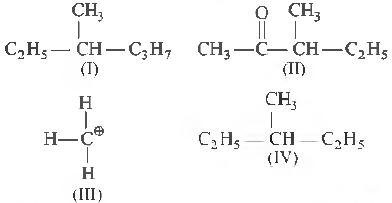
it is true that
(a) all fow- are chiral compounds
(b) I and II are chiral compounds
(c) only ill is a chiral compound
(d) IT and IV are chiral compounds
Answer
B
Question 15. The hybridisation of carbon atom in C — C single bond of H2C = CH — CH = CH2 is
(a) sp3 — sp
(b) sp2 — sp
(c) sp2 — sp2
(b) sp3 — sp3
Answer
C
Question 16. The functional group present in organic, acid is –
(a) – OH
(b) – CHO
(c) –COOH
(d) > C = O
Answer
C
Question 17. Which of these contains the carbonyl group?
(a) ketones
(b) aldehydes
(c) esters
(d) all of these
Answer
D
Question 18. Which of the following is incorrectly matched –
(a) vinegar →carboxylic acid
(b) C2H6 →alkane
(c) ethanol →alcohol
(d) methanol →ketone
Answer
D
Question 19. Amongst the following compounds, the optically active alkane having lowest molecular mass is
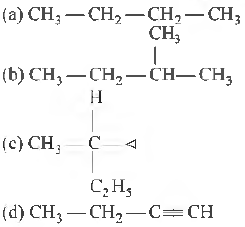
Answer
C
Question 20. The functional group present in CH3COOC2H5 is –
(a) ketonic
(b) aldehydic
(c) ester
(d) carboxylic
Answer
C
Question 21. Which of the following have incorrect molecular formula?
A. Icosane – C10H22
B. Triacontane – C30H62
C. Nonane – C9H20
D. Heptane – C7H14
(a) (A) and (D)
(b) Only (D)
(c) (B) and (D)
(d) Only (B)
Answer
A
Question 22. Butanone is a four-carbon compound with the functional group –
(a) carboxylic acid
(b) aldehyde.
(c) ketone
(d) alcohol.
Answer
C
Question 23. The compound, whose stereo-chemical fommla is written below, exhibits x geometrical isomers and y optical isomers The values of x and y are
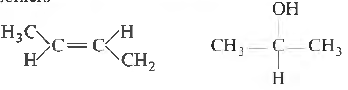
(a) 4 and 4
(b) 2 and 2
(c) 2 and 4
(d) 4 and 2
Answer
B
Question 24. Which of the following statements is false for isopentane ?
(a) It has three CH3 groups
(b) It has one CH2 group
(c) It has one CH group
(d) It has a carbon which is not bonded to hydrogen
Answer
D
Question 25. Geometrical isomerism is possible in
(a) acetone oxime
(b) isobutene
(c) acetophenone oxime
(d) benzophenone oxime
Answer
C
Question 26. The compound which has one isopropyl group is
(a) 2, 2, 3, 3 – Tetramethylpentane
(b) 2, 2 – Dimethylpentane
(c) 2, 2, 3- Trimethylpentane
(d) 2- Methypentane
Answer
D
Question 27.

Compound can exhibit
(a) geometrical isomerism
(b) tautomerism
(c) optical isomerism
(d) geometrical and optical isomerism
Answer
C
Question 28. In this reaction,
CH3CHO+HCN → CH3CH(OH)CN
→H·OH CH3CH(OH)COOH
an asymmetric centre is generated. The acid obtained would be
(a) 50% D + 50% L-isomer
(b) 20% D + 80% L-isomer
(c) D-isomer
(d) L-isomer
Answer
A
Question 29. Which one of the following shows functional isomerism?
(a) C2H4
(b) C3H6
(c) C2H5OH
(d) CH2Cl2
Answer
C
Question 30. Ethers are isomeric with
(a) aldehydes
(b) ketones
(c) both aldehydes and ketones
(d) alcohols
Answer
D
Question 31. Which of the following will have a meso-isomer also?
(a) 2-chlorobutane
(b) 2, 3-dichlorobutane
(c) 2, 3-dichloropentane
(d) 2-hydroxypropanoic acid
Answer
B
Question 32. Which of the following compounds is optically active ?
(a) (CH3 )2CHCH2OH
(b) CH3CH2OH
(c) CCI2F2
(d) CH3CHOHC2H5
Answer
D
Question 33. Isomers of prop ionic acid are
(a) HCOOC2H5 and CH3COOCH3
(b) HCOOCC2H5 and C3H7COOH
(c) CH3COOCH3 and C3H7OH
(d) C3H7OH and CH3COOCH3
Answer
A
Question 34. The name of the compound

is
(a) (2Z, 4Z)-2, 4-hexadiene
(b) (2Z, 4E)-2, 4-hexadiene
(c) (2E, 4Z)-2, 4-hexadiene
(d) (4E, 4Z)-2, 4-hexadiene
(e) (2E, 4E)-2, 4-hexadiene
Answer
A
Question 35. Geometrical isomerism is shown by
(a) —C—C—
(b) C=C
(c) — C- C—
(d) None of these
Answer
B
Question 36. n-pentane, iso-pentane and neo-pentane are examples for isomers of the type
(a) geometrical
(b) optical
(c) chain
(d) positional
Answer
C
Question 37. Different structures generated due to rotation about, C—C axis, of an organic molecule, are examples of
(a) geometrical isomerism
(b) conformational isomerism
(c) optical isomerism
(d) structural isomerism
Answer
B
Question 38. Which of the following Fischer’s projection fommla is identical to D-glyceraldehyde?
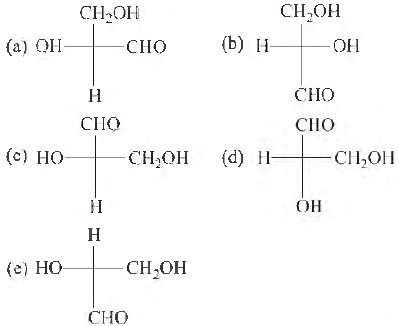
Answer
B
Question 39. Metamers of ethyl propionate are
(a) C4H9COOH and HCOOC4H9
(b) C4H9COOH and CH3COOC3H7
(c) CH3COOCH3 and CH3COOC3H7
(d) CH3COOC3H7 and C3H7COOCH3
Answer
D
Question 40. Increasing order of stability among the three main conformations, (i.e. Eclipse, Anti, Gauche) of 2-fluoroethanol is
(a) Eclipse, Gauche, Anti
(b) Gauche, Eclipse, Anti
(c) Eclipse, Anti, Gauche
(d) Anti, Gauche, Eclipse
Answer
C
Whoever needs to take the CBSE Class 11 Board Exam should look at this MCQ. To the Students who will show up in CBSE Class 11 Chemistry Board Exams, It is suggested to practice more and more questions. Aside from the sample paper you more likely had solved. These Organic Chemistry Class 11 MCQ are ready by the subject specialists themselves.
Question 41. How many asymmetric carbon atoms are present in
I. 1,2-dimethylcyclohexane
II. 3-methylcyclopentane and
III. 3-methylcyclohexene ?
(a) Two, one, one
(b) One, one, one
(c) Two, none, two
(d) Two, none, one
Answer
D
Question 42. For which of the following parameters the structural isomers C2H5OH and CH3OCH3 would be expected to have the same values? (Assume ideal behaviour)
(a) Heat of vaporisation
(b) Vapour pressure at the same temperature
(c) Boiling points
(d) Gaseous densities at the same temperature and pressure
Answer
D
Question 43. Maximun1 enol content is in
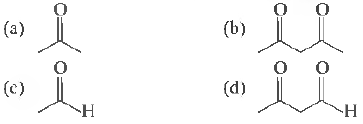
Answer
B
Question 44. C6H5C = N and C6H5N = C exhibit which type of isomerism?
(a) Position
(b) Functional
(c) Metamerism
(d) Dextro-isomerism
Answer
B
Question 45. How many isomers are possible for the alkane C4H10 ?
(a) 3
(b) 5
(c) 2
(d) 4
Answer
C
Question 46. Geometrical isomerism is possible in case of
(a) pentene-2
(b) propane
(c) pentane
(d) ethene
Answer
A
Question 47. The number of isomers of the compound with molecular fommla C2H2Br2 is
(a) 4
(b) 3
(c) 5
(d) 2
Answer
D
Question 48. The number of primary, secondary and tertiary carbons in 3, 4-dimethylheptane are respectively
(a) 4, 3 and 2
(b) 2, 3 and 4
(c) 4, 2 and 3
(d) 3, 4 and 2
Answer
A
Question 49. The number of primary, secondary, tertiary and quaternary carbons in neopentane are respectively
(a) 4, 3, 2 and 1
(b) 5, 0, 0 and 1
(c) 4, 0, 0 and 1
(d) 4, 0, 1 and 1
Answer
C
Question 50. The number of meso-forms in the following compound is
HOOC- CH(CH3 )· CH(OH)· CH(Cl)
· CH(OH)CH(CH3 )· COOH
(a) 3
(b) 4
(c) 8
(d) 16
Answer
B
Question 51. Which of the following compounds is optically active ?
(a) 1-butanol
(b) Isopropyl alcohol
(c) Acetaldehyde
(d) 2-butanol
Answer
D
Question 52. The number of secondary hydrogens in 2, 2-dimethylbutane is
(a) 8
(b) 6
(c) 4
(d) 2
Answer
D
Question 53. What is the IUPAC name of t-butyl alcohol.
(a) Butanol–2
(b) 2–Methyl-propan–2-ol
(c) Butanol–1
(d) Propanol-2
Answer
B
Question 54. Which of the following compounds contains 1°, 2°, 3° as well as 4° carbon atoms ?
(a) Neopentane
(b) 2-methyl pentane
(c) 2,3-dimethyl butane
(d) 2,2,3-trimethyl pentane
Answer
D
Question 55. The IUPAC name of neopentane is
(a) 2, 2-dimethylpropane
(b) 2-methylpropane
(c) 2, 2-dimethylbutane
(d) 2-methylbutane
Answer
A
Question 56. The IUPAC name of the compound CH3 — CH(CH3) — CO – CH3, is
(a) 3-methyl 2-butanone
(b) 2-methyl 3-butanone
(c) isopropyl methyl ketone
(d) methyl isopropyl ketone
Answer
A
Question 57. The IUPAC name of CH3COCH (CH3)2 is –
(a) isopropyl methyl ketone
(b) 2-methyl-3-butanone
(c) 4-methylisopropyl ketone
(d) 3-methyl-2-butanone
Answer
D
Question 58. IUPAC name of (CH3)3 CCl is
(a) 1-butyl chloride
(b) 3-chloro butane
(c) 2-chloro-2-methylpropane
(d) 2-butyl chloride
Answer
C
Question 59. The IUPAC name of the compound CH3OCH2CH2CH2OCH2CH3 is
(a) 3-ethoxy-1-methoxypropane
(b) 1-ethoxy-3-methoxypropane
(c) 2, 5-dioxyhexane
(d) ethoxypropane oxymethane
Answer
A
Question 60. Which organic structure among the following is not an isomer of the compound CH3–CO–CH2CH2CH2CH3 ?
(a) CH3CH2OCH =CHCH2CH3
(b) CH3CH = CHCH2CH2CHO
(c) (CH3)2CH–CO–CH2CH3
(d) CH3CH2COCH2CH2CH3
Answer
B
Question 61. Total number of structural isomers possible for C3H6 are :
(a) 2
(b) 1
(c) 4
(d) 3
Answer
A
Question 62. Which of the following statements is false for isopentane–
(a) It has three CH3 groups
(b) It has one CH2 group
(c) It has one CH group
(d) It has a carbon which is not bonded to hydrogen
Answer
D
Question 63. The total number of isomers for C4H8 is
(a) 5
(b) 6
(c) 7
(d) 8
Answer
B
Question 64. The least number of carbon atoms in alkane showing isomerism is
(a) 3
(b) 1
(c) 2
(d) 4
Answer
D
Question 65. The number of possible alkynes with molecular formula C5H8 is
(a) 2
(b) 3
(c) 4
(d) 5
Answer
B
Question 66. Which of the following compounds is isomeric with 2, 2, 4, 4- tetramethylhexane?
(a) 3-ethyl -2, 2- dimethylpentane
(b) 4-isopropylheptane
(c) 4-ethyl-3-methyl-4-n propyloctane
(d) 4, 4-diethyl-3-methylheptane
Answer
B
Question 67. Isomers of propionic acid are
(a) HCOOC2H5 and CH3COOCH3
(b) HCOOC2H5 and C3H7COOH
(c) CH3COOCH3 and C3H7OH
(d) C3H7OH and CH3COCH3
Answer
A
Question 68. An aromatic compound of formula C7H7Cl has in all ….. isomers :
(a) 5
(d) 2
(c) 4
(d) 3
Answer
C
Question 69. CH3CH2OH and CH3OCH3 are the examples of
(a) chain isomerism
(b) functional isomerism
(c) position isomerism
(d) metamerism
Answer
B
Question 70. In which of the following, functional group isomerism is not possible?
(a) Alcohols
(b) Aldehydes
(c) Alkyl halides
(d) Cyanides
Answer
C
Question 71. Which are isomers ?
(a) ethyl alcohol and dimethyl ether
(b) acetone and acetaldehyde
(c) propionic acid and propanone
(d) methyl alcohol and dimethyl ether
Answer
A
Question 72. C6H5C ≡ N and C6H5N ≡ C are which type of isomers?
(a) Position
(b) Functional
(c) Tautomerism
(d) Linkage
Answer
B
Question 73. A functional isomer of 1-butyne is
(a) 2-butyne
(b) 1-butene
(c) 2-butene
(d) 1, 3-butadiene
Answer
D
Question 74. Methoxyethane and propanol are the examples of isomerism of the type
(a) structural
(b) position
(c) functional
(d) tautomerism
Answer
C
Question 75. The compounds CH3CH == CHCH3 and CH3CH2CH == CH2
(a) are tautomers
(b) are position isomers
(c) contain same number of sp3– sp3, sp3– sp2 and sp2– sp2 carbon-carbon bonds
(d) exist together in dynamic equilibrium
Answer
B
Question 76. The organic reaction which proceed through heterolytic bond cleavage are called ________
(a) ionic
(b) polar
(c) nonpolar
(d) Both (a) and (b)
Answer
D
Question 77. The shape of methyl carbanion is similar to that of –
(a) BF3
(b) NH3
(c) methyl free radical
(d) methyl carbocation
Answer
B
Question 78. Heterolytic fission of C – Br bond results in the formation of
(a) free radical
(b) carbanion
(c) carbocation
(d) Both (b) and (c)
Answer
C
Question 79. Which of the following carbocations is least stable?
(a) tert-Alkyl
(b) sec-Alkyl
(c) pri-Alkyl
(d) Methyl
Answer
D
You can easily get good marks If you study with the help of Class 11 Organic Chemistry MCQ. We trust that information provided is useful for you. NCERT Organic Chemistry Class 11 MCQ PDF Free Download would without a doubt create positive results.
We hope the information shared above in regards to MCQ on Organic Chemistry Class 11 with Answers has been helpful to you. if you have any questions regarding CBSE Class 11 Chemistry Solutions MCQs Pdf, write a comment below and we will get back to you as soon as possible.
Frequently Asked Question (FAQs)
How many MCQ questions are there in Class 11 chapter 12 Chemistry?
In Class 11 Chapter 12 Chemistry, we have provided 79 Important MCQ Questions, But in the future, we will add more MCQs so that you can get good marks in the Class 11 exam.
Can we score good marks in Class 11 Chemistry with the help of Organic Chemistry MCQ Questions?
Yes, MCQ Question is one of the best strategies to make your preparation better for the CBSE Board Exam. It also helps to know the student’s basic understanding of each chapter. So, You can score good marks in the Class 11 Chemistry exam.