Class 12 Chemical Kinetics MCQs are one of the best strategies to prepare for the CBSE Class 12 Board exam. If you want to complete a grasp concept or work on one’s score, there is no method except constant practice. Students can improve their speed and accuracy by doing more Class 12 MCQ on Chemical Kinetics pdf with answers which will help them all through their board test.
Class 12 Chemical Kinetics MCQ Questions with Answers
Class 12 Chemistry MCQ with answers are given here to chapter Chemical Kinetics. These MCQs are based on the latest CBSE board syllabus and relate to the latest Class 12 Chemistry syllabus. By Solving these Class 12 MCQs, you will be able to analyze all of the concepts quickly in the chapter and get ready for the Class 12 Annual exam.
Learn Chemical Kinetics Class 12 MCQ with answers pdf free download according to the latest CBSE and NCERT syllabus. Students should prepare for the examination by solving Class 12 Chemical Kinetics MCQ with answers given below.
Question 1. For a reaction between A and B the order with respect to A is 2 and the order with respect to B is 3.
The concentrations of both A and B are doubled, the rate will increase by a factor of
(a) 12
(b) 16
(c) 32
(d) 10
Answer
C
Question 2. The decomposition of phosphine (PH3) on tungsten at low pressure is a first-order reaction. It is because the
(a) rate is proportional to the surface coverage
(b) rate is inversely proportional to the surface coverage
(c) rate is independent of the surface coverage
(d) rate of decomposition is very slow.
Answer
A
Question 3. The rate constant of the reaction A B is 0.6 × 10–3 mol L–1 s–1. If the concentration of A is 5 M, then concentration of B after 20 minutes is
(a) 3.60 M
(b) 0.36 M
(c) 0.72 M
(d) 1.08 M
Answer
C
Question 4. Which one of the following statements for the order of a reaction is incorrect?
(a) Order can be determined only experimentally.
(b) Order is not influenced by stoichiometric coefficient of the reactants.
(c) Order of a reaction is sum of power to the concentration terms of reactants to express the rate of reaction.
(d) Order of reaction is always whole number.
Answer
D
Question 5. The unit of rate constant for a zero order reaction is
(a) mol L–1 s–1
(b) L mol–1 s–1
(c) L2 mol–2 s–1
(d) s–1
Answer
A
Question 6. In a reaction, A + B → product, rate is doubled when the concentration of B is doubled, and rate increases by a factor of 8 when the concentration of both the reactants (A and B) are doubled, rate law for the reaction can be written as
(a) rate = k[A][B]2
(b) rate = k[A]2[B]2
(c) rate = k[A][B]
(d) rate = k[A]2[B]
Answer
D
Question 7. If the rate of the reaction is equal to the rate constant, the order of the reaction is
(a) 0
(b) 1
(c) 2
(d) 3
Answer
A
Question 8. For the reaction, A + B → products, it is observed that
(i) on doubling the initial concentration of A only, the rate of reaction is also doubled and
(ii) on doubling the initial concentration of both A and B, there is a change by a factor of 8 in the rate of the reaction.
The rate of this reaction is given by
(a) rate = k[A][B]2
(b) rate = k[A]2[B]2
(c) rate = k[A][B]
(d) rate = k[A]2[B]
Answer
A
Question 9. The rate of reaction between two reactants A and B decreases by a factor of 4 if the concentration of reactant B is doubled. The order of this reaction with respect to reactant B is
(a) 2
(b) –2
(c) 1
(d) –1
Answer
B
Question 10. For the reaction; 2N2O5 → 4NO2 + O2 rate and rate constant are 1.02 × 10–4 and 3.4 × 10–5 sec–1 respectively, then concentration of N2O5 at that time will be
(a) 1.732
(b) 3
(c) 1.02 × 10–4
(d) 3.4 × 105
Answer
B
Question 11. The given reaction,
2FeCl3 + SnCl2 → 2FeCl2 + SnCl4 is an example of
(a) third order reaction
(b) first order reaction
(c) second order reaction
(d) none of these.
Answer
A
Question 12. 2A B → C, It would be a zero order reaction when
(a) the rate of reaction is proportional to square of concentration of A
(b) the rate of reaction remains same at any concentration of A
(c) the rate remains unchanged at any concentration of B and C
(d) the rate of reaction doubles if concentration of B is increased to double.
Answer
B
Question 13. A first order reaction has a rate constant of 2.303 × 10–3 s–1. The time required for 40 g of this reactant to reduce to 10 g will be [Given that log10 2 = 0.3010]
(a) 230.3 s
(b) 301 s
(c) 2000 s
(d) 602 s
Answer
D
Question 14. The rate constant for a first order reaction is 4.606 × 10–3 s–1. The time required to reduce 2.0 g of the reactant to 0.2 g is
(a) 100 s
(b) 200 s
(c) 500 s
(d) 1000 s
Answer
C
Question 15. If the rate constant for a first order reaction is k, the time (t) required for the completion of 99% of the reaction is given by
(a) t = 2.303/k
(b) t = 0.693/k
(c) t = 6.909/k
(d) t = 4.606/k
Answer
D
Question 16. When initial concentration of the reactant is doubled, the half-life period of a zero order reaction
(a) is halved
(b) is doubled
(c) is tripled
(d) remains unchanged.
Answer
B
Question 17. A first order reaction has a specific reaction rate of 10–2 sec–1. How much time will it take for 20 g of the reactant to reduce to 5 g?
(a) 138.6 sec
(b) 346.5 sec
(c) 693.0 sec
(d) 238.6 sec
Answer
A
Question 18. The correct difference between first and second order reactions is that
(a) the rate of a first-order reaction does not depend on reactant concentrations; the rate of a second-order reaction does depend on reactant concentrations
(b) the half-life of a first-order reaction does not depend on [A]0 ; the half-life of a second-order reaction does depend on [A]0
(c) a first-order reaction can be catalysed; a secondorder reaction cannot be catalysed
(d) the rate of a first-order reaction does depend on reactant concentrations; the rate of a secondorder reaction does not depend on reactant concentrations.
Answer
B
Question 19. A reaction is 50% complete in 2 hours and 75% complete in 4 hours. The order of reaction is
(a) 1
(b) 2
(c) 3
(d) 0
Answer
A
Question 20. The rate of first-order reaction is 0.04 mol L–1 s–1 at 10 seconds and 0.03 mol L–1 s–1 at 20 seconds after initiation of the reaction. The half-life period of the reaction is
(a) 44.1 s
(b) 54.1 s
(c) 24.1 s
(d) 34.1 s
Answer
C
Question 21. When initial concentration of a reactant is doubled in a reaction, its half-life period is not affected. The order of the reaction is
(a) second
(b) more than zero but less than first
(c) zero
(d) first.
Answer
D
Question 22. Half-life period of a first order reaction is 1386 seconds. The specific rate constant of the reaction is
(a) 0.5 × 10–2 s–1
(b) 0.5 × 10–3 s–1
(c) 5.0 × 10–2 s–1
(d) 5.0 × 10–3 s–1
Answer
B
Question 23. If 60% of a first order reaction was completed in 60 minutes, 50% of the same reaction would be completed in approximately (log 4 = 0.60,log 5 = 0.69)
(a) 45 minutes
(b) 60 minutes
(c) 40 minutes
(d) 50 minutes.
Answer
A
Question 24. The half-life of a substance in a certain enzymecatalysed reaction is 138 s. The time required for the concentration of the substance to fall from 1.28 mg L–1 to 0.04 mg L–1 is
(a) 414 s
(b) 552 s
(c) 690 s
(d) 276 s
Answer
C
Question 25. The reaction A B follows first order kinetics. The time taken for 0.8 mole of A to produce 0.6 mole of B is 1 hour. What is the time taken for conversion of 0.9 mole of A to produce 0.675 mole of B ?
(a) 1 hour
(b) 0.5 hour
(c) 0.25 hour
(d) 2 hours
Answer
A
Question 26. For a first order reaction A B the reaction rate at reactant concentration of 0.01 M is found to be 2.0 × 10–5 mol L–1 s–1. The half-life period of the reaction is
(a) 30 s
(b) 220 s
(c) 300 s
(d) 347 s
Answer
D
Question 27. The rate of a first order reaction is 1.5 × 10–2 mol L–1 min–1 at 0.5 M concentration of the reactant. The half-life of the reaction is
(a) 0.383 min
(b) 23.1 min
(c) 8.73 min
(d) 7.53 min
Answer
B
Question 28. A plot of 1/ T versus k for a reaction gives the slope – 1 x 104 K. The energy of activation for the reaction is (Given, R = 8.314K-1 mol-1)
(a) 83.14 J mol-1
(b) 1.202 kJ mol-1
(c) 12.02 J mol-1
(d) 83.14 kJ mo1-1
Answer
D
Question 29. The rate constant (k1 ) of one of the reaction is found to be double that of the rate constant (k2 ) of another reaction. The relationship between the corresponding activation energies of the two reactions Ea1 and Ea2 will be
(a) Ea1 < Ea2
(b) Ea1 > Ea2
(c) Ea1 = Ea2
(d) Ea1 = 2Ea2
Answer
A
Question 30. Raw milk sours in about 4 hat 27°C, but in about 48 h in a refrigerator at 17°C. What is the activation energy for souring of milk?
(a) 78.0 kl mol-1
(b) 46.21 kl mol-1
(c) 23.5 kl mol-1
(d) 80.8 kl mol-1
Answer
A
Whoever needs to take the CBSE Class 12 Board Exam should look at this MCQ. To the Students who will show up in CBSE Class 12 Chemistry Board Exams, It is suggested to practice more and more questions. Aside from the sample paper you more likely had solved. These Class 12 Chemical Kinetics MCQs PDF are ready by our subject specialists themselves.
Question 31. A chemical reaction was carried out at 300 K and 280 K. The rate constants were found to be k1 and k2 respectively. Then
(a) k2 ≈ 0.25 k1
(b) k2 ≈0.5 k1
(c) k2 ≈ 4 k1
(d) k2 ≈ 2 k1
Answer
A
Question 32. For a reaction taking place in three steps, the rate constants are k1 , k2 and k3 and overall rate constant is k = k1k3 / k2 . If the energies of activation E1, E2 and E3 are 60, 30 and 10 kJ mol-1 , respectively, then the overall energy of activation is
(a) 30 kJ mol-1
(b) 40 kJ mol-1
(c) 60 kJ mol-1
(d) l00 kJ mol-1
Answer
B
Question 33. The rate ofreaction doubles when its temperature changes from 300 K to 310 K. Activation energy of such a reaction will be(R = 8.314 JK-1 mol-1 and log 2 = 0.301)
(a) 53.6 kJ mol-1
(b) 48.6 kJ mol-1
(c) 58.5 kJ mol-1
(d) 60.5 kJ mol-1
Answer
A
Question 34. A given sample of milk turns sour at room temperature (27°C) in 5 h. In a refrigerator at -3° C., it can be stored 10 times longer. The energy of activation for the souring of milk is
(a) 2.303 x 5 R kJ mol-1
(b) 2.303 x 3 R kJ mol-1
(c) 2.303 x 2.7 R kJ mol-1
(d) 2.303 x 10 R kl mol-1
Answer
C
Question 35. The rate of a reaction is doubled for every 10° rise in temperature. The increase in reaction rate as a result of temperature rise from 10° to 100° is
(a) 112
(b) 512
(c) 400
(d) 614
(e) 100
Answer
B
Question 36. N2 (g) + 2H2 (g) ⇌ 2NH3 (g) + 22 kcal
The activation energy for the forward reaction is 50 kcal. What is the activation energy for the backward reaction?
(a) 72 kcal
(c) -72 kcal
(b) 28 kcal
(d) -28kcal
Answer
A
Question 37. A reactant ( A ) forms two products

If ![]() then
then ![]() and
and ![]() are related as
are related as
(a) ![]()
(b)![]()
(c)![]()
(d)![]()
Answer
B
Question 38. The rate of a chemical reaction doubles for every 10°C rise of temperature. If the temperature is raised by 50°C., the rate of the reaction increases by about
(a) IO times
(b) 24 times
(c) 32 times
(d) 64 times
Answer
C
Question 39. In the given graph the activation energy, Ea for the reverse reaction will be

(a) 50 kJ
(b) 50 kJ
(c) 200 kJ
(d) 100 kJ
Answer
B
Question 40. The activation energy of a reaction at a given temperature is found to be 2.303 RT J mol-1. The ratio of rate constant to the Arrhenius factor is
(a) 0.01
(b) 0.1
(c) 0.02
(d) 0.001
Answer
B
Question 41. The activation energy of exothermic reaction A ➔ B 80 kJ mol-1. The heat of reaction is 200 kJ mol-1. The activation energy for the reaction B ➔ A (in kJ mol-1) will be
(a) 80
(b) 120
(c) 40
(d) 280
Answer
D
Question 42. Plots showing the variation of the rate constant ( k) with temperature (T) are given below. The plot that follows Arrhenius equation is

Answer
A
Question 43. For a first order reaction A ➔ P, the temperature (T) dependent rate constant ( k) was found to follow the equation log k = – (2000)/ T + 6.0 The pre-exponential factor A and the actjvation energy Ea, respectively, are
(a) 1.0 x 106s-1 and 9.2 kJ mol-1
(b) 6.0s-1 and 16.6 kJ mol-1
(c) 1.0 x 106s-1 and 16.6 kJ mol-1
(d) 1.0 x 106s-1 and 38.3 kJ mol-1
Answer
D
Question 44. By increase in temperature by 10 K, the rate of reaction becomes double. How many times the rate of reaction will be if the temperature is increased from 303K to 353 K?
(a) 4
(b) 8
(c) 16
(d) 32
Answer
D
Question 45. If k1 = rate constant at temperature T1 and k2 = rate constant at temperature T2 for a first order reaction, then which of the following relations is correct?
(Ea : activation energy)
(a)![]()
(b)![]()
(c)![]()
(d)![]()
Answer
B
Question 46. The activation energies of two reactions are E1 and E2 (E1 > E ). If the temperature of the system is increased from T1 to T2 , the rate constant of the reactions changes from k1 to k2 in the first reaction and k2 to k2 in the second reaction. Preruct which of the following expression is correct ?
(a) k1 / k1 = k2 / k2
(b) k1 / k1 > k2 / k2
(c) k1 / k1 < k2 / k2
(d) k1 / k1 = k2 / k2 = 1
(e) k1 / k1 = k2 / k2 = 0
Answer
B
Question 47. Which of the following statement is in accordance with collision theory ?
I. Rate is directly proportional to collision frequency
II. Rate depends upon orientation of atoms
III. Temperature determines the rate
(a) Only III
(b) Only I and II
(c) Only II and III
(d) All of these
Answer
D
Question 48. If Xis the total number of collisions which a gas molecule register with others per unit time under particular conditions, then the collision frequency of the gas containing N molecules per unit volume is
(a) X / N
(b) NX
(c) 2 NX
(d) NX / 2
Answer
D
Question 49. For a first-order reaction, the half-life period is independent of
(a) first power of final concentration
(b) cube root of initial concentration
(c) initial concentration
(d) square root of final concentration.
Answer
C
Question 50. For a reversible reaction, A ⇌ B,
which one of the following statements is wrong from the given energy profile diagram ?
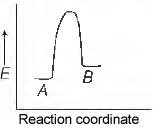
(a) Activation energy of forward reaction is greater than backward reaction
(b) The forward reaction is endothermic
(c) The threshold energy is less than that of activation energy
(d) The energy of activation of forward reaction is equal to the sum of heat of reaction and the energy of activation of backward reaction
Answer
C
Question 51. What is the activation energy for a reaction if its rate doubles when the temperature is raised from 20 °C to 35 °C? (R = 8.314 J mol–1 K–1)
(a) 34.7 kJ mol–1
(b) 15.1 kJ mol–1
(c) 342 kJ mol–1
(d) 269 kJ mol–1
Answer
A
Question 52. For a reaction, activation energy Ea = 0 and the rate constant at 200 K is 1.6 × 106 s–1. The rate constant at 400 K will be [Given that gas constant R = 8.314 J K–1 mol–1]
(a) 3.2 × 104 s–1
(b) 1.6 × 106 s–1
(c) 1.6 × 103 s–1
(d) 3.2 × 106 s–1
Answer
B
Question 53. The addition of a catalyst during a chemical reaction alters which of the following quantities?
(a) Enthalpy
(b) Activation energy
(c) Entropy
(d) Internal energy
Answer
B
Question 54. The rate of the reaction,
2NO + Cl2 → 2NOCl is given by the rate equation, rate = k[NO]2[Cl2]. The value of the rate constant can be increased by
(a) increasing the temperature
(b) increasing the concentration of NO
(c) increasing the concentration of the Cl2
(d) doing all of these.
Answer
A
Question 55. The activation energy for a simple chemical reaction A ⇌ B is Ea in forward direction. The activation energy for reverse reaction
(a) is negative of Ea
(b) is always less than Ea
(c) can be less than or more than Ea
(d) is always double of Ea.
Answer
C
Question 56. In a zero-order reaction, for every 10 °C rise of temperature, the rate is doubled. If the temperature is increased from 10 °C to 100 °C, the rate of the reaction will become
(a) 256 times
(b) 512 times
(c) 64 times
(d) 128 times.
Answer
B
Question 57. Activation energy of a chemical reaction can be determined by
(a) evaluating rate constants at two different temperatures
(b) evaluating velocities of reaction at two different temperatures
(c) evaluating rate constant at standard temperature
(d) changing concentration of reactants.
Answer
A
Question 58. When a biochemical reaction is carried out in laboratory, outside the human body in absence of enzyme, then rate of reaction obtained is 10–6 times, the activation energy of reaction in the presence of enzyme is
(a) 6/RT
(b) P is required
(c) different from Ea obtained in laboratory
(d) can’t say anything.
Answer
C
Question 59. How enzymes increases the rate of reactions?
(a) By lowering activation energy
(b) By increasing activation energy
(c) By changing equilibrium constant
(d) By forming enzyme substrate complex
Answer
A
Question 60. An increase in the concentration of the reactants of a reaction leads to change in
(a) activation energy
(b) heat of reaction
(c) threshold energy
(d) collision frequency.
Answer
D
Question 61. By the action of enzymes, the rate of biochemical reaction
(a) does not change
(b) increases
(c) decreases
(d) either (a) or (c).
Answer
B

You can easily get good marks If you study with the help of Class 12 Chemical Kinetics MCQs. We trust that information provided is useful for you. NCERT MCQ Questions for Class 12 Chemical Kinetics PDF Free Download would without a doubt create positive results.
We hope the information shared above in regards to Class 12 MCQ on Chemical Kinetics pdf with answers has been helpful to you. if you have any questions regarding CBSE Chemical Kinetics Class 12 MCQ PDF, write a comment below and we will get back to you as soon as possible.
Frequently Asked Question (FAQs)
How many MCQ questions are there in Class 12 Chemistry Chapter 4?
In Class 12 Chemistry Chapter 4, we have provided 61 Important MCQ Questions, But in the future, we will add more MCQs so that you can get good marks in the Class 12 exam.
Can we score good marks in Class 12 Chemistry with the help of Chemical Kinetics MCQ Questions?
Yes, MCQ Question is one of the best strategies to make your preparation better for the CBSE Board Exam. It also helps to know the student’s basic understanding of each chapter. So, You can score good marks in the Class 12 Chemistry exam.

